The Food and Drug Administration on Thursday approved Bristol Myers Squibb’s highly anticipated schizophrenia drug, Cobenfy, marking the first novel type of treatment for this debilitating mental disorder in over 70 years.
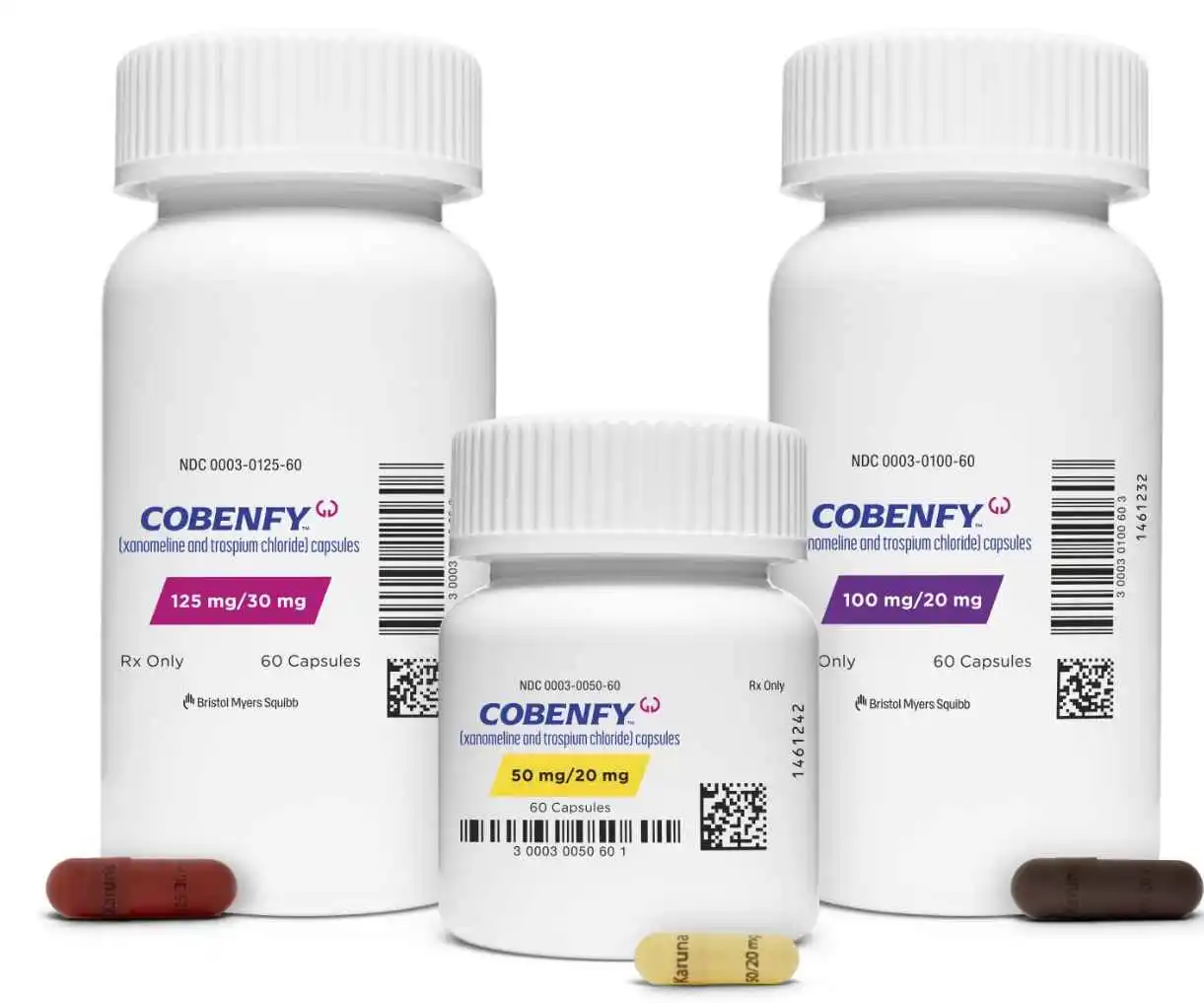
This approval represents a significant advancement in the management of schizophrenia, offering new hope to patients and healthcare providers alike.

The Burden of Schizophrenia: A Persistent Challenge
Schizophrenia is a chronic mental disorder that profoundly affects how a person thinks, feels, and behaves. It can lead to a range of symptoms, including paranoia, delusions, hallucinations, and noticeable changes in emotions, movements, and behavior.
These symptoms often disrupt a patient’s daily life, making it challenging to attend school or work, engage in social activities, and perform routine tasks. Most individuals are diagnosed in their late teens to early 30s, a critical period for personal and professional development.
Despite the availability of treatments, managing schizophrenia remains a significant challenge. According to Bristol Myers Squibb, nearly 3 million adults in the U.S. live with schizophrenia, yet only 1.6 million receive treatment.
Alarmingly, 75% of these patients discontinue their medications within the first 18 months, primarily due to ineffective treatments or intolerable side effects.
Cobenfy: A Novel Approach to Schizophrenia Treatment
Cobenfy stands out as the first treatment approved from a new class of drugs that do not directly block dopamine receptors to alleviate schizophrenia symptoms. Instead, it offers a different approach by targeting muscarinic receptors in the brain.
Dr. Samit Hirawat, Bristol Myers Squibb’s Chief Medical Officer, explained, “Cobenfy’s mechanism involves activating certain muscarinic receptors, which in turn decreases dopamine activity without causing the side effects commonly associated with traditional antipsychotics.”

The Dual-Action Mechanism: Xanomeline and Trospium
Cobenfy is a twice-daily oral medication composed of two active components:
- Xanomeline: This drug activates specific muscarinic receptors in the brain. By stimulating these receptors, xanomeline indirectly modulates dopamine pathways, helping to reduce psychotic symptoms without directly blocking dopamine receptors.
- Trospium: This component serves to mitigate the gastrointestinal side effects associated with xanomeline, such as nausea, vomiting, diarrhea, and constipation. Trospium does not cross the blood-brain barrier, ensuring it does not interfere with xanomeline’s therapeutic effects on the central nervous system.
Clinical Trials: Efficacy and Safety Profile
The FDA’s approval was based on data from three pivotal clinical trials comparing Cobenfy to a placebo, alongside two long-term studies assessing safety and tolerability over one year.
The results were promising:
- Promising Results: Symptom Reduction and Tolerability. Cobenfy met the primary endpoints in all three trials, significantly decreasing the symptoms of schizophrenia compared to placebo. Patients exhibited improvements in both positive symptoms (like hallucinations and delusions) and negative symptoms (such as social withdrawal and apathy).
- Onset of Action: Some patients reported noticeable improvements within weeks of starting treatment, suggesting a relatively rapid therapeutic effect.
- Safety: The most common adverse events were mild to moderate gastrointestinal issues, primarily nausea, vomiting, and constipation. Importantly, these side effects tended to diminish over time and were manageable for most patients.
- Addressing Common Side Effects of Traditional Psychotics: Unlike many traditional antipsychotics, Cobenfy was not associated with significant weight gain, excessive fatigue, or involuntary muscle movements (extrapyramidal symptoms). This favorable side effect profile may enhance patient adherence and overall quality of life.
Dr. Andrew Miller, founder and former president of research and development at Karuna Therapeutics (now an advisor to Bristol Myers Squibb), emphasized the drug’s potential impact:
“I think there’s potentially a really transformational moment in how we treat and talk about schizophrenia. We’ve helped people, we’ve improved outcomes, and we’ve provided caregivers and physicians with another tool that they can use.”
Implications for Clinical Practice
For healthcare providers, Cobenfy offers a new therapeutic option that may address some of the limitations of existing treatments:
- A New Option for Treatment-Resistant Patients: Approximately one-third of individuals with schizophrenia do not respond adequately to conventional antipsychotics. Cobenfy’s novel mechanism provides an alternative pathway to symptom relief for these patients.
- Reduced Side Effects: The avoidance of direct dopamine blockade may reduce the incidence of metabolic and neurological side effects, which are significant factors in medication discontinuation.
- Potential for Improved Medication Adherence: A more tolerable side effect profile can lead to better adherence, a critical component in the long-term management of schizophrenia.
Adam Lenkowsky, Bristol Myers Squibb’s Chief Commercialization Officer, noted, “The enthusiasm we’re hearing from physicians is the opportunity to have a patient go onto treatment without seeing the side effects but also getting unprecedented efficacy.”
Beyond Schizophrenia: Exploring Future Applications
Cobenfy’s approval for schizophrenia may be just the beginning. Bristol Myers Squibb is exploring its potential in other neuropsychiatric conditions:
- Alzheimer’s Disease Psychosis: Psychotic symptoms are common in Alzheimer’s disease, contributing to increased caregiver burden and accelerated cognitive decline. Cobenfy’s muscarinic receptor activity could offer benefits in this population. Clinical trials are underway, with data expected in 2026.
- Bipolar Mania: The drug is being studied for its potential to manage manic episodes in bipolar disorder, which can include symptoms like elevated mood, hyperactivity, and delusions.
- Irritability in Autism Spectrum Disorder: Research is planned to assess Cobenfy’s effectiveness in reducing irritability and aggression in individuals with autism.
Dr. Hirawat expressed optimism about these future indications:
“When we think about Cobenfy, we think about it as multiple indications packed in one product because we are really developing the drug not only for schizophrenia but six other indications.”
The Significance of Cobenfy’s Novel Mechanism
The development of Cobenfy underscores the importance of exploring new pathways in the treatment of complex mental health disorders.
Traditional antipsychotics have relied on dopamine receptor antagonism, a mechanism associated with numerous side effects that can limit their utility.
By targeting muscarinic receptors, Cobenfy opens a new avenue for symptom management. This approach may pave the way for additional therapies that can address unmet needs in psychiatry.
Cobenfy’s targeting of muscarinic receptors is significant because it introduces a novel mechanism for treating schizophrenia, distinct from traditional antipsychotics that block dopamine receptors.
By activating specific muscarinic receptors in the brain, Cobenfy can reduce psychotic symptoms without the common side effects associated with dopamine antagonists, such as weight gain, sedation, and involuntary movements.
This new approach not only offers effective symptom management but also improves tolerability, potentially enhancing patient adherence and overall outcomes in schizophrenia treatment.
Expert Perspectives: A Game-Changing Treatment?
Psychiatric experts have highlighted the potential of Cobenfy:
- Dr. Joshua Kantrowitz, Director of the Columbia Schizophrenia Research Center, stated that Cobenfy is both highly effective at treating symptoms without the serious side effects of existing treatments. “If further studies conclusively show such a benefit, then it’s getting to the point where it could be a true game-changer,” he said.
- Andrea Cipriani, Psychiatry Professor at the University of Oxford, noted that while clinical rating tools show promise, it’s essential to see how these translate into real-world effects. She emphasized the need for ongoing research to fully understand Cobenfy’s impact.
Looking Ahead: A Transformational Moment in Psychiatry
The approval of Cobenfy represents more than just a new medication; it symbolizes hope for a patient population that has long faced limited options and significant stigma. Schizophrenia not only affects individuals but also has profound societal consequences, including homelessness and encounters with law enforcement.
Gordon Lavigne, CEO of the Schizophrenia & Psychosis Action Alliance, remarked, “There haven’t been breakthroughs, new innovations in the treatment of schizophrenia in a really, really long time. Cobenfy is game-changing, potentially.”
Conclusion: A New Era in Schizophrenia Treatment
Cobenfy’s approval marks a significant milestone in the field of psychiatry. As the first novel treatment for schizophrenia in decades, it offers a new mechanism of action that may improve patient outcomes and quality of life. For doctors and pharmacists, it provides an additional tool in the therapeutic arsenal against a challenging and complex disorder.
As we look to the future, the ongoing research into Cobenfy’s applications in other conditions may further expand its impact. The potential to treat Alzheimer’s disease psychosis, bipolar mania, and autism-related irritability highlights the versatility of this novel approach.
Dr. Miller aptly summarized the significance: “The most important moment is going to be five or ten years from now when we look back and say we’ve actually made a difference. We’ve helped people, we’ve improved outcomes, we’ve provided caregivers and physicians with another tool that they can use.”
Guidance for Healthcare Professionals
As with any new medication, it’s crucial to review the full prescribing information for Cobenfy and consider individual patient needs when integrating it into treatment plans. Ongoing monitoring and collaboration with patients will be essential to maximize benefits and minimize risks.