Uzbekistan is strengthening its pharmaceutical industry by focusing on the development of locally produced blood plasma-derived medicines.
The government has implemented legislative changes and investment plans to modernize the country’s blood collection, processing, and plasma-derived medicine production capabilities.

Investment Plans for Blood Collection, Processing, and Plasma-Derived Medicine Production
The government has recently implemented legislative amendments and investment plans to modernize and expand the country’s blood collection, processing, and plasma-derived medicine production capabilities.
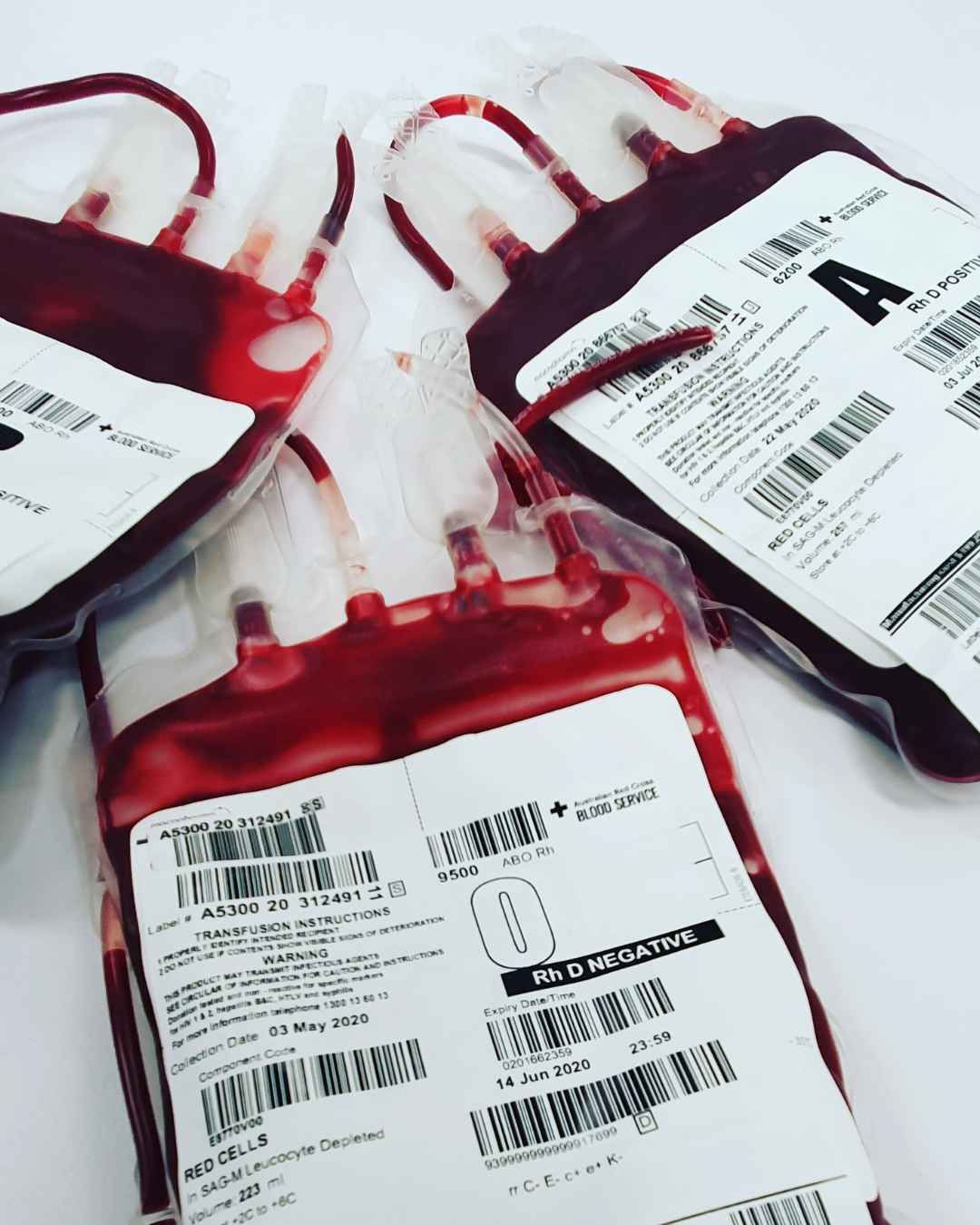
These changes aim to strengthen the domestic pharmaceutical industry, reduce reliance on imports, and improve the availability of essential medicines for the population.
Pharma Investment Opportunities and Development Plans
Uzbekistan has outlined a list of prospective investment projects in the pharmaceutical industry that require the development of project documentation between 2022 and 2026.
One of these projects involves setting up the production of medicines derived from blood plasma, indicating the government’s intention to expand the country’s manufacturing capacity for plasma-derived pharmaceutical products.
These investment plans are part of a broader effort to develop and strengthen the domestic pharmaceutical industry in Uzbekistan.
Rafarma Pharmaceuticals: Plans Blood Plasma Collection and Production in Uzbekistan
Rafarma Pharmaceuticals, Inc., a company with a long-term presence in the Central Asian market, has expressed interest in expanding its operations in Uzbekistan.
The company plans to increase its presence by creating local production facilities within the innovative research and production pharmaceutical cluster “Tashkent Pharma Park”.
Over the next three years, Rafarma aims to build a plant designed according to Industry 4.0 standards, featuring a flexible industrial base that can easily, quickly, and cost-effectively undergo significant modernization and modification depending on production tasks, drugs, and required technologies.
The plant will include two production units:
- Production of sterile preparations for the market of Uzbekistan and the countries of Central Asia
- Production of fractionated blood plasma elements and other products in the form of frozen plasma and blood factors, as well as the development of a network of laboratories for plasma procurement
Two-Stage Project: Certified Laboratories Network and Plasma Fractionation Plant
Rafarma’s project, which is part of a list of measures to accelerate the development of the pharmaceutical industry in Uzbekistan in 2022-2026, will consist of two stages:
- Creation of a modern certified laboratories network for plasma collection
- Creation of a plant for plasma fractionation
The company expects to generate revenue in the tens of millions of dollars within 4-5 years of implementing this project.
The Uzbekistan government is currently working on legislative changes to the principles of working with blood plasma in the medical sector and preparing documents to allow the export of medicines based on blood plasma.
Rafarma will begin implementing its project as soon as the necessary regulatory framework is created by the government, which is expected to happen soon.
HaemaTech Expands Blood Plasma Operations in Uzbekistan
HaemaTech Limited, a Hong Kong based blood products and plasma company, is significantly expanding its operations in Uzbekistan. The company currently operates four blood collection centers in the capital city of Tashkent that employ around 100 people.
Looking to further grow its presence, HaemaTech signed an investment agreement with Uzbekistan’s Pharmaceutical Industry Development Agency earlier this year.
As part of the agreement, HaemaTech will build a major new blood products manufacturing facility at the Tashkent Pharma Park pharmaceutical research cluster. This new plant is expected to create an additional 600 jobs.
Furthermore, HaemaTech has plans to establish additional blood collection centers across Uzbekistan in the future to support the increased production.
Regulatory Framework for Blood Plasma-Derived Medicines
Presidential Decree Enabling Private Sector Participation
In January 2022, Presidential Decree No. UP-55 lifted the ban on the private sector’s involvement in the collection of donor blood and the production of pharmaceutical products derived from it.
This decision paved the way for further legislative changes to create a legal framework for private organizations to participate in these activities.
Licensing, GMP Certification, and Quality Standards for Plasma-Derived Medicines
On April 22, 2024, the Cabinet of Ministers issued Resolution No. 225, which approved the Regulation on the Procedure for Organizing the Production of Medicines from Donor Blood Plasma.
This regulation sets out the requirements for legal entities engaged in the extraction, collection, production, storage, and delivery of medicines from donor blood plasma, including:
- Obtaining appropriate licenses for medical and pharmaceutical activities
- Acquiring a certificate of compliance with the national requirements of Good Manufacturing Practice (GMP)
- Using registered medical equipment for plasmapheresis to extract plasma as a drug substance
The resolution also outlines separate requirements for achieving quality in the production of medicines from donor blood.