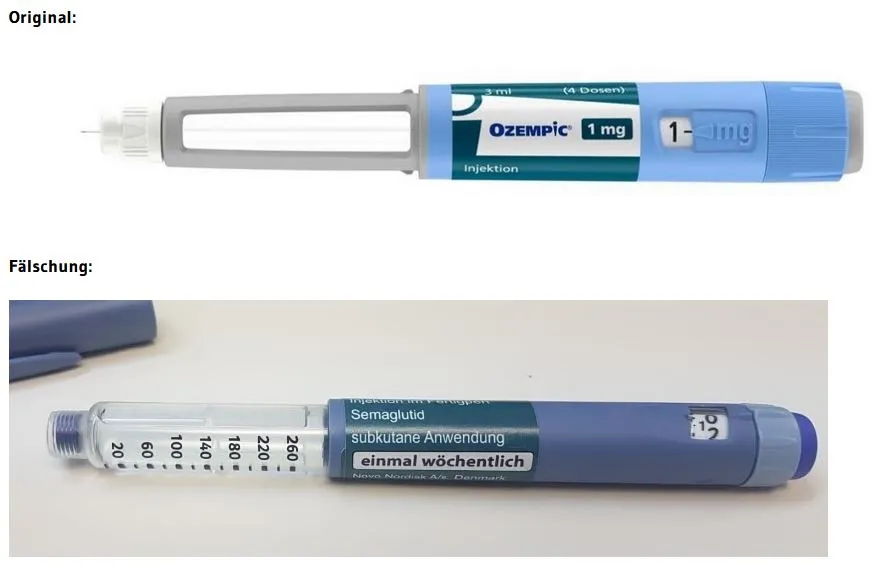
The European Medicines Agency has published information for patients and healthcare professionals on the discovery of falsely labeled pre-filled adulterated Ozempic® (semaglutide, 1 mg, solution for injection) syringe pens from EU and UK distributors.
The pens with German language labels came from wholesalers in Austria and Germany and feature lot numbers, 2D barcodes and the unique serial numbers of the original Ozempic® packages.
The counterfeits were detected thanks to the electronic pharmaceutical traceability system that operates in the EU. When the packages of counterfeit Ozempic® were scanned, the serial numbers were inactive and the operator was alerted to potential counterfeiting.
Regulators in Germany and Austria have issued notices to wholesalers for failing to comply with good distribution practices.
The EMA recommends that patients study the Ozempic® Ozempic® information leaflet and pay attention to how the original Ozempic® pens should look. If the appearance of the pen is suspicious, it should not be used, as this may lead to serious health consequences. Such a pen should be returned to the pharmacy immediately. Do not buy the medicine on the Internet, it should be purchased only in legal pharmacies.
Wholesalers and pharmacies are advised to exercise caution when purchasing Ozempic from suppliers. Any suspicion should be reported to the national competent authority. However, pharmacists should check pens when dispensing and advise patients how to identify adulterated packages (based on the patient information leaflet and photographs published by the German Medicines Agency). Health care providers should also remind patients that medicines should only be purchased from legal pharmacies.
Images of original and counterfeit drug Ozempic® (Source: Federal Institute of Drugs and Medical Devices – BfArM).



