

On August 21, 2024, the Ukrainian parliament introduced new legislation that formalizes the off-label use of registered medicinal products.
This regulation establishes clear and structured procedures for healthcare professionals (HCPs) to prescribe medicines for non-authorized indications, age groups, dosages, or methods of administration—collectively known as off-label use.
For Ukrainian doctors and our colleagues across the CIS region, this development represents both progress and a call for balanced discretion in clinical practice.
Legalizing Off-Label Prescriptions
Historically, off-label prescribing has been an unofficial yet common practice in Ukraine.
Physicians have often resorted to prescribing medications beyond their approved indications to address complex patient needs, especially in areas with limited treatment options. Pediatric indications are missing from many medicines, though often prescribed.
While this approach demonstrated clinical ingenuity, it operated in a legal gray area, potentially exposing both patients and practitioners to unforeseen risks.
The new legislation moderates this landscape by providing a legal framework that prioritizes patient safety, transparency, and accountability.
By formalizing off-label use, Ukraine aligns itself with international best practices, fostering a more reliable and standardized approach to patient care.
Key Amendments: Ensuring Safety and Accountability
The new law introduces several critical amendments aimed at regulating off-label prescriptions effectively:

Patient Safety and Informed Consent
Before prescribing off-label, HCPs must provide patients—or their legal representatives—with comprehensive information about the risks, expected benefits, alternative treatment options, and available clinical trials in Ukraine.
This ensures that patients are fully informed and can make educated decisions regarding their treatment.

Eligibility Criteria for Off-Label Use
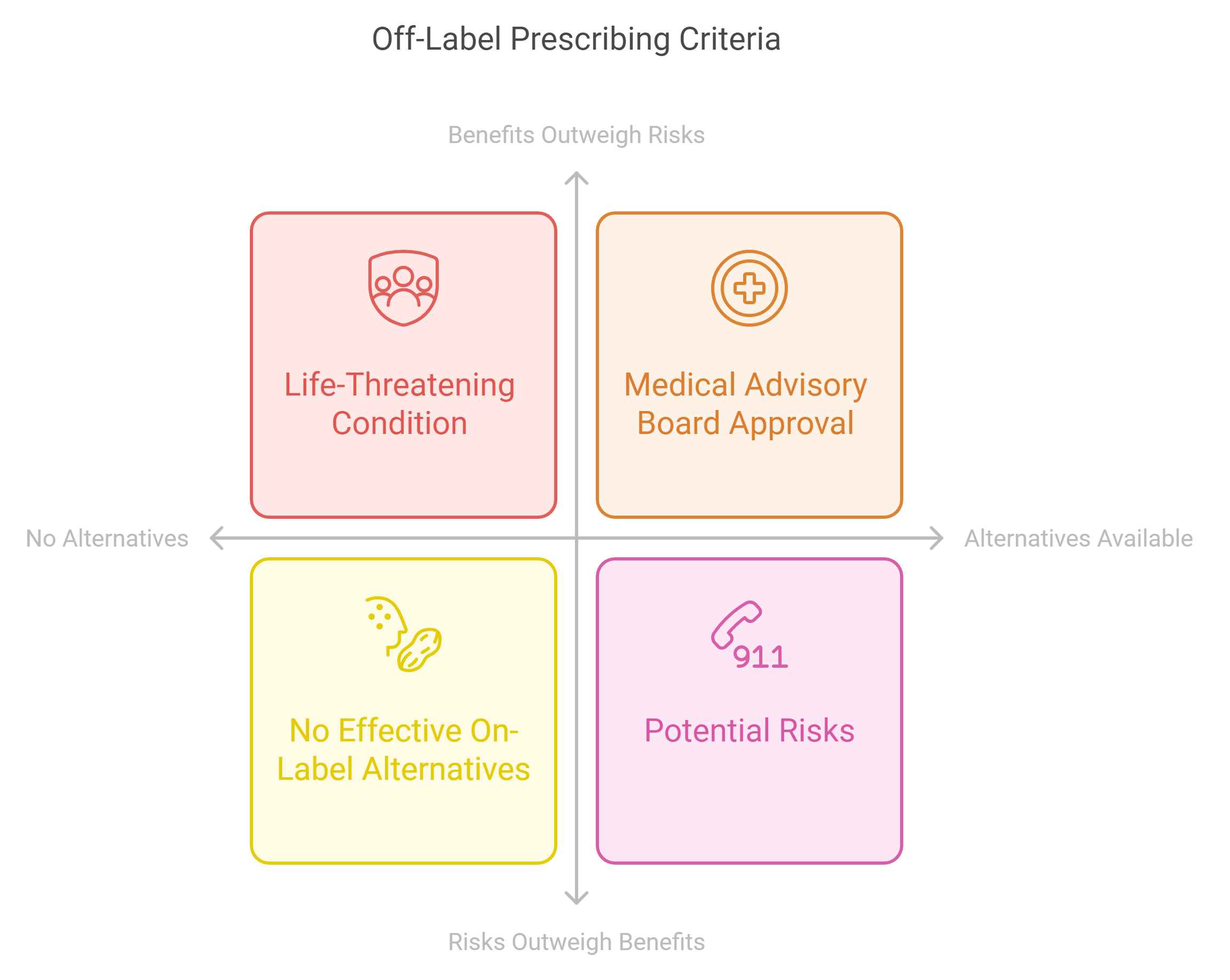
Off-label prescribing is permissible only under stringent conditions:
- The patient is suffering from a life-threatening condition or a disease significantly impairing their quality of life.
- No approved treatment alternatives are available in Ukraine that meet the patient’s needs.
- The expected benefits of the off-label medicine outweigh the potential risks.
- A medical advisory board within the healthcare institution must confirm the absence of effective on-label alternatives and recommend off-label use.
Documentation and Medical Justification
Every off-label prescription must be meticulously documented in the patient’s medical records, including:
- The rationale behind the off-label use.
- References to relevant clinical guidelines, including foreign ones, as permitted.
- The formal decision from the medical advisory board, detailing the involved HCPs and their consensus.
Balancing Regulation with Clinical Discretion
While the new regulations are a commendable step toward standardized care, some aspects may impose additional burdens on practitioners.
The requirement for medical advisory board approval and extensive documentation, although essential for accountability, can potentially slow down the decision-making process in urgent clinical scenarios.
In the United States, the off-label prescribing process is more liberal, allowing physicians greater discretion to tailor treatments based on individual patient needs without mandatory advisory board approval. Doctors make their own decisions, without committee discussions.
This flexibility can be crucial in fast-paced or critical care environments where timely decisions are paramount.
Ukrainian doctors may find the new procedures more stringent, yet they provide a working framework that enhances patient safety and treatment transparency.
However, there is a growing discussion that the law could benefit from incorporating more flexibility, empowering doctors to act swiftly in the best interests of their patients without compromising on safety and accountability.
Aligning with International Standards
Ukraine’s legislation echoes the European Union’s principles regarding off-label use, especially in emphasizing patient safety and informed consent.
Moreover, the structured documentation and justification process introduced by Ukraine surpasses the requirements in some EU member states, ensuring a higher standard of regulatory compliance.
Implementation Timelines
The new law is poised to take effect immediately following its official publication, with enforceability commencing three months thereafter. The formal publication is anticipated within the coming weeks, with full enactment expected by the end of 2024.
This phased approach allows healthcare institutions ample time to adapt to the new regulations and integrate them into their clinical workflows.
Implications for Reimbursement and Procurement
Importantly, the legislation does not automatically facilitate the reimbursement or procurement of off-label medicines.
Historically, off-label procurement has occurred, such as in oncology treatments for pediatric patients without specific indications.
Under the new law, centralized procurement agencies—including those of the Ministry of Health, regional governments, or hospitals—can procure therapies for off-label use.
However, individual patient prescriptions must adhere to the new procedures, necessitating medical advisory board approval and informed consent.
Looking Ahead: Advocacy for Balanced Regulation
This is a legislative advancement, however it is crucial for the medical community to engage in ongoing dialogue with policymakers. After the law goes into force, it is very likely that many specific objections will be made to how the law was written, or now being interpreted.
Hopefully legislators and authorities will be open minded to further improve the legislation.
Advocating for a balanced regulatory environment that safeguards patient interests while granting physicians the necessary discretion can ensure that off-label prescribing remains a viable and effective component of clinical practice.
Off label is a proven, standard practice in many countries, Ukraine just needs to find and strike a logical balance.




