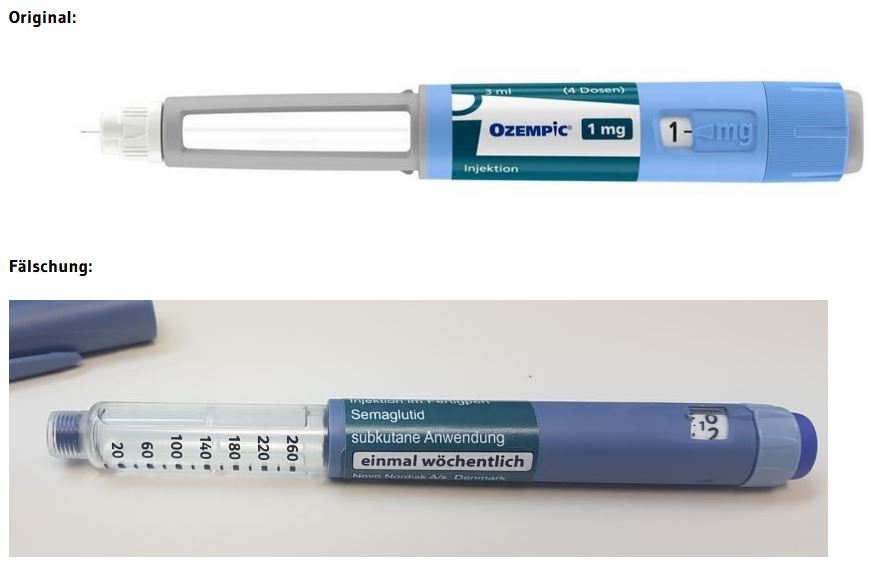
The hospital in Zaporizhzhya suspended the heads of two departments, whose patients complained about the purchase of medicines at their own expense
If a medical institution fails to purchase necessary medicines or consumables, and patients are forced to buy them at their own expense, appropriate management decisions will be taken on the management staff of such hospitals. The same applies to doctors who prescribe medicines to patients without proven efficacy, which the latter also buy at their own expense